Biomimetic Total Synthesis
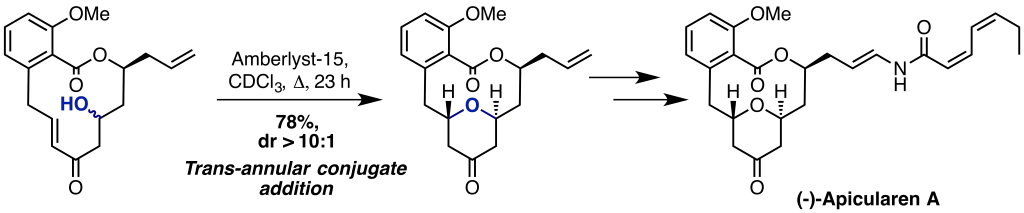
Upon the isolation and characterisation of natural products, it is always interesting to try and understand the biogenesis of these complex architectures, and applying this process in a laboratory setting. The group has been successful in applying biomimetic chemistry to the total synthesis of complex natural products. Recent examples include our synthesis of (-)-Apicularen A, which utilised a key trans-annular conjugate addition to yield the tetrahydropyran ring.1
Our total synthesis of Silvestrol made use of an elegant biomimetic [3+2] cycloaddition to yield the cyclopenta[b]benzofuran core of silvestrol in high yield over 3 steps.2
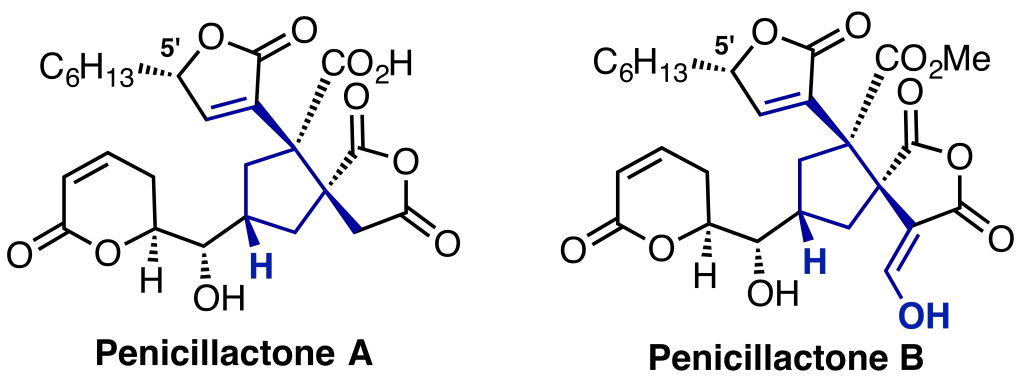
Looking at the Penicillactone family of natural products, we believe the spiro[5.5]nonane core, can be synthesised in the lab through a biomimetic synthesis. A total synthesis of these natural products will also allow for confirmation of the stereochemistry at C5′.
(1) F. Hilli, J. M. White, and M. A. Rizzacasa, Org. Lett., 2004, 6, 1289–1292. F. Hilli, J. M. White, and M. A. Rizzacasa, Tetrahedron, 2011, 67, 5054–5068.(2) , , G. A. Holloway, D. J. Owen, P. J. Scammells, and M. A. Rizzacasa, Angew. Chem. Int. Ed., 2007, 46, 7835–7838, T. E. Adams, M. El Sous, B. C. Hawkins, S. Hirner, G. A. Holloway, M. L. Khoo, D. J. Owen, G. P. Savage, P. J. Scammells, and M. A. Rizzacasa, J. Am. Chem. Soc., 2009, 131, 1607–1616.