Development of new Asymmetric Catalysis
In our formal total synthesis of (-)-Spirangien A,1 a key step was the catalytic Mukaiyama hydration reaction, on the complex enone 1 to yield α-hydroxyketone 2. This reaction utilised an easily synthesised catalyst, cheap reagents, and molecular O2 to furnish 2. This operationally simple procedure came with one drawback, and that is the lack of stereocontrol at C20, which resulted in two epimers, dramatically reducing the yield of the desired enone.
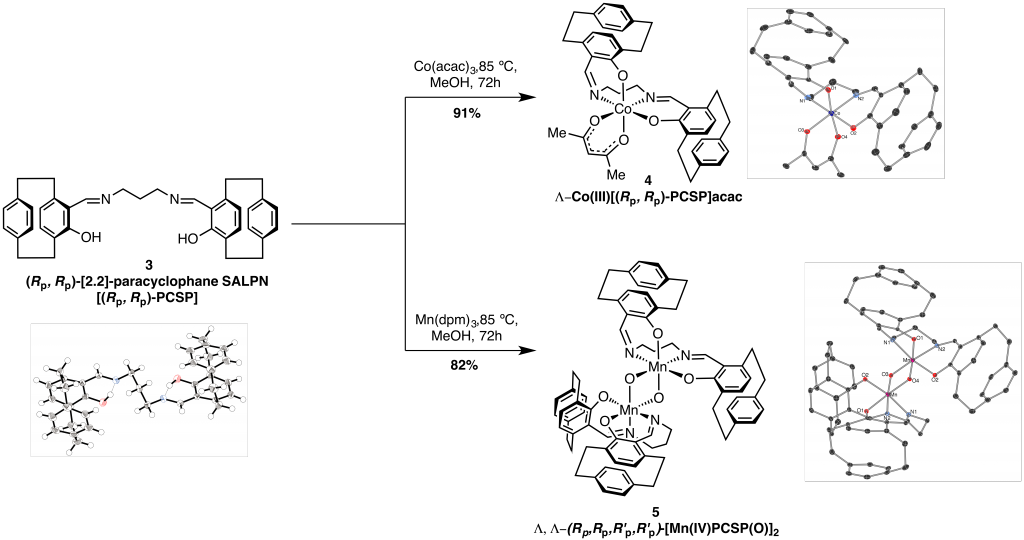
Recently our efforts have been focussed on addressing this limitation, leading to the synthesis of chiral metal octahedral complexes with the goal of effecting this important transformation asymmetric.2
We have synthesised two novel metal complexes with [2.2]-paracyclophane salen ligand 3. Upon complexeation with CoIII and MnIII with 3, the planar chirality within ligand 3, is transferred upon the metal centre resulting in cis-β octahedral complexes CoIII 4 and MnIV 5 complexes with Λ absolute stereochemistry at the metal centre.
Unfortunately these catalysts were unable to catalyse the Mukayama hydration reaction, our group is in the process of identifying alternative catalysts for this reaction.
(1) C. Gregg, C. Gunawan, A. W. Y. Ng, S. Wimala, S. Wickremasinghe, and M. A. Rizzacasa, Org. Lett., 2013, 15, 516-519. (2) D. Loits, S. Bräse, A. J. North, J. M. White, P. S. Donnelly, and M. A. Rizzacasa, Eur. J. Inorg. Chem., 2016, 2016, 3541–3544.