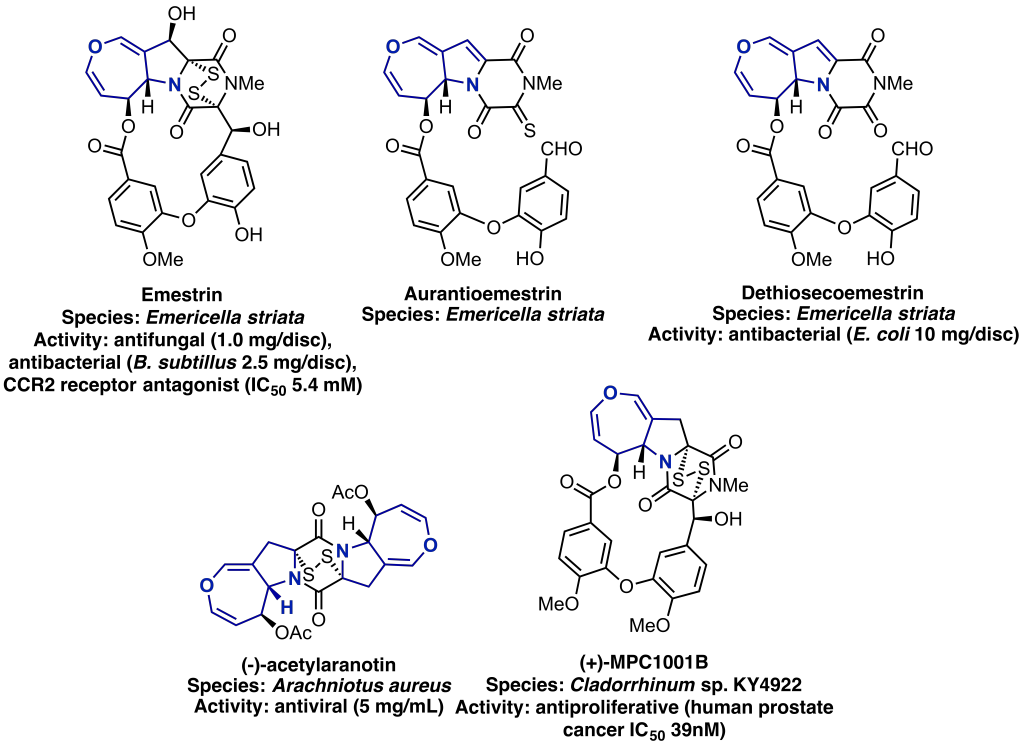
Total Synthesis of Thioketopiperazine Natural Products
Antimicrobial resistance is a global threat, if left unaddressed, superbugs may kill an estimated 10 million people a year by 2050. One avenue of research is the isolation and identification of secondary metabolites that could potentially become antibacterials of the future. Thioketopiperazine natural products are secondary metabolites, isolated form a range of fungal species, as well as exhibiting a range of biological activity including antibacterial and antifungal properties, their complex structural motifs are make them attractive synthetic targets.
Common to this family of natural products is the dihydrooxepino[4,3-b]pyrrole core, to which we required a quick and efficient synthetic route, if we are to access meaningful quantities of the final natural products. To this end we developed a unique route, which includes a vinylogous Darzens between a suitable protected pyrrole aldehyde and ethyl bromocrotonate, to yield the highly substituted vinyl pyrrole epoxide as a single diastereoisomer. Finally by simply heating this this pyrrole epoxide in CCl4, it undergoes a [3,3] sigmatropic rearrangement to yield the dihydrooxepino[4,3-b]pyrrole core in excellent yield.1
(1). A. Cameron, B. Fisher, N. Fisk, J. Hummel, J. M. White, E. H. Krenske, and M. A. Rizzacasa, Org. Lett., 2015, 17, 5998–6001.