Publications
2016
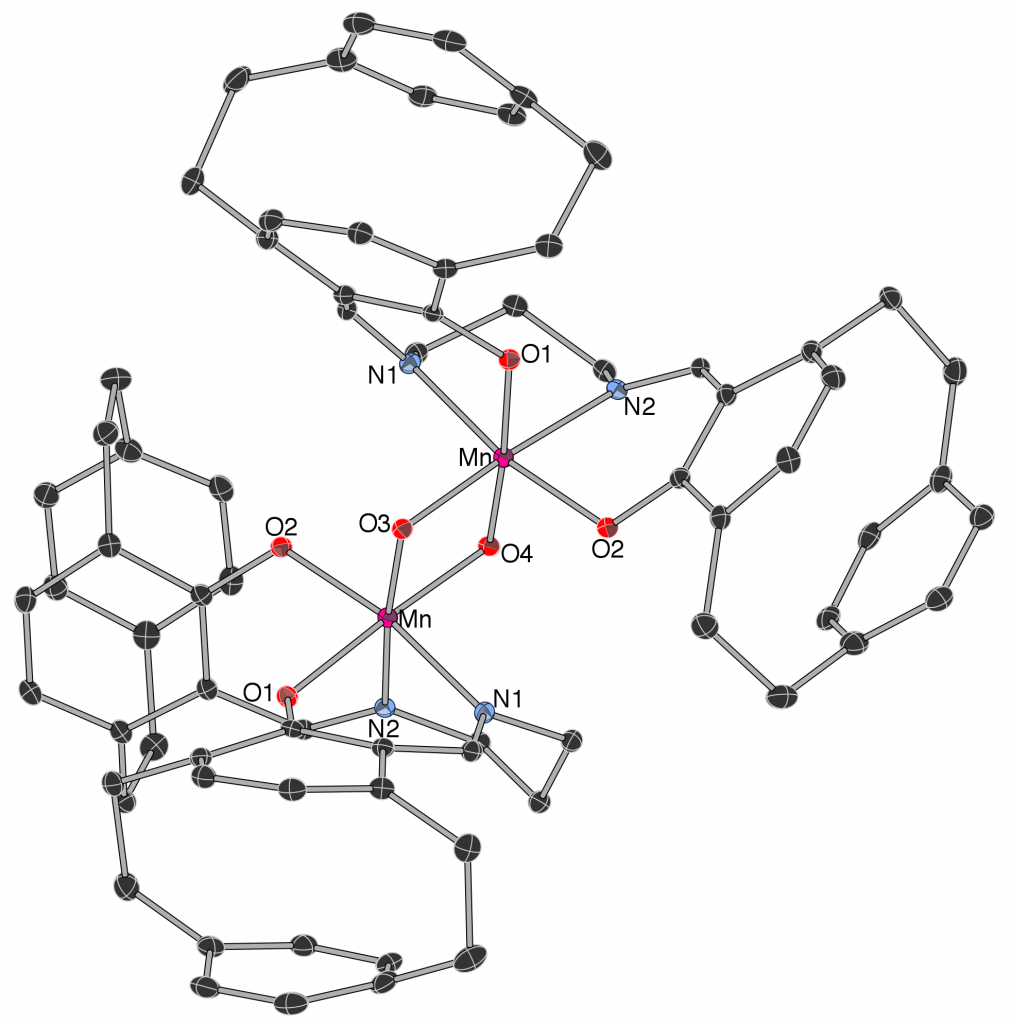
75. D. Loits, S. Bräse, A. J. North, J. M. White, P. S. Donnelly, and M. A. Rizzacasa, ‘Synthesis of Homochiral Co III – and Mn IV -[2.2]Paracyclophane Schiff Base Complexes with Predetermined Chirality at the Metal Centre’, Eur. J. Inorg. Chem., 2016, 2016, 3541–3544.
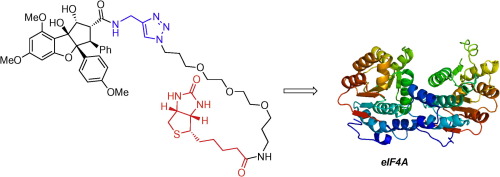
74. J. M. Chambers, L. M. Lindqvist, G. P. Savage, and M. A. Rizzacasa, ‘Total synthesis of a biotinylated rocaglate: Selective targeting of the translation factors eIF4AI/II’, Bioorg. Med. Chem. Lett., 2016, 26, 262–264.
2015
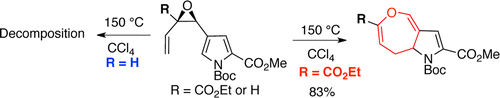
73. A. Cameron, B. Fisher, N. Fisk, J. Hummel, J. M. White, E. H. Krenske, and M. A. Rizzacasa, ‘Towards the Synthesis of Dihydrooxepino[4,3-b]pyrrole-Containing Natural Products via Cope Rearrangement of Vinyl Pyrrole Epoxides’, Org. Lett., 2015, 17, 5998–6001.
2014
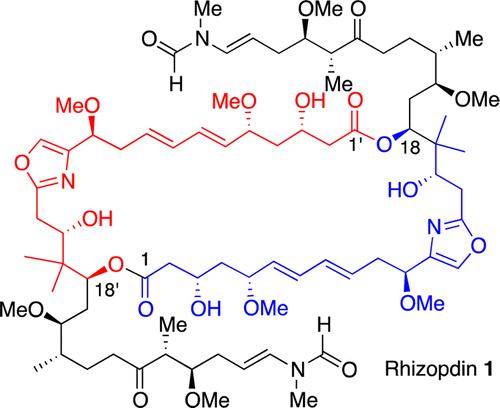
72. T. Bender, D. Loits, J. M. White, and M. A. Rizzacasa, ‘Synthesis of the C1–C18 Fragment of Rhizopodin: Late-State Introduction of the Oxazole’, Org. Lett., 2014, 16, 1450–1453.
71. N. Al-Zaubai, C. N. Johnstone, M. M. Leong, J. Li, M. Rizzacasa, and A. G. Stewart, ‘Resolvin D2 Supports MCF-7 Cell Proliferation via Activation of Estrogen Receptor’, J. Pharmacol. Exp. Ther., 2014, 351,172–180.
70. B. C. Hawkins, L. M. Lindqvist, D. Nhu, P. P. Sharp, D. Segal, A. K. Powell, M. Campbell, E. Ryan, J. M. Chambers, J. M. White, M. A. Rizzacasa, G. Lessene, D. C. S. Huang, and C. J. Burns, ‘Simplified Silvestrol Analogues with Potent Cytotoxic Activity’, ChemMedChem, 2014, 9, 1556–1566.
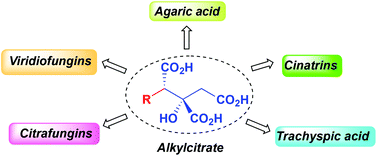
69. M. A. Rizzacasa and D. Sturgess, ‘Total synthesis of alkyl citrate natural products’, Org. Biomol. Chem., 2014, 12, 1367-1382.
2013
68. C. Gregg, C. Gunawan, A. W. Y. Ng, S. Wimala, S. Wickremasinghe, and M. A. Rizzacasa, ‘Formal Total Synthesis of Spirangien A’, Org. Lett., 2013, 15, 516–519.
67. J. M. Chambers, L. M. Lindqvist, A. Webb, D. C. S. Huang, G. P. Savage, and M. A. Rizzacasa, ‘Synthesis of Biotinylated Episilvestrol: Highly Selective Targeting of the Translation Factors eIF4AI/II’, Org. Lett., 2013, 15,1406–1409.
66. S. Birkett, D. Ganame, B. C. Hawkins, S. Meiries, T. Quach, and M. A. Rizzacasa, ‘Total Synthesis of the Proposed Structure of 8-Deshydroxyajudazol A: A Modified Approach to 2,4-Disubstituted Oxazoles’, J. Org. Chem., 2013, 78,116–123.
65. J. Li, M. M. Leong, A. Stewart, and M. A. Rizzacasa, ‘Total synthesis of the endogenous inflammation resolving lipid resolvin D2 using a common lynchpin’, Beilstein J. Org. Chem., 2013, 9, 2762–2766.
2012
64. M. A. Rizzacasa, D. Balan, C. J. Burns, N. Fisk, H. Hügel, D. C. S. Huang, D. Segal, and J. Wagler, ‘Synthesis and Biological Evaluation of a Potent Salicylihalamide A Lactam Analogue’, Org. Biomol. Chem., 2012, 10, 8147–8153.
63. S. L. Birkett, D. A. Loits, S. Wimala, and M. A. Rizzacasa, ‘Synthesis of myxobacteria metabolites’, Pure Appl. Chem., 2012, 84,1421–1433.
62. M. A. Rizzacasa, M. Morozova, and S. Wickremasinghe, ‘Synthesis of Novel Oligosaccharides Based on 1,4-Dioxanyloxy 3-Oxasugars’, Heterocycles, 2012, 84, 1067.
61. S. Boonsri, C. Gunawan, E. H. Krenske, and M. A. Rizzacasa, ‘Synthetic studies towards the mulberry Diels-Alder adducts: H-bond accelerated cycloadditions of chalcones’, Org. Biomol. Chem., 2012, 10, 6010–6021.
60. J. M. Chambers, D. C. S. Huang, L. M. Lindqvist, G. P. Savage, J. M. White, and M. A. Rizzacasa, ‘Total Synthesis of 2’”,5”’-Diepisilvestrol and Its C1”’ Epimer: Key Structure Activity Relationships at C1”’ and C2”’’, J. Nat. Prod., 2012, 75,1500–1504.
2011
59. F. Hilli, J. M. White, and M. A. Rizzacasa, ‘Formal total synthesis of the myxobacteria metabolite apicularen A via a transannular oxy-Michael addition’, Tetrahedron, 2011, 67, 5054–5068.
58. M. A. Rizzacasa, M. Morozova, and S. Wickremasinghe, ‘Synthesis of Novel Oligosaccharides Based on 1,4-Dioxanyloxy 3-Oxasugars’, Heterocycles, 2011, 84, 1067–1079.
57. S. Birkett, D. Ganame, B. C. Hawkins, S. Meiries, T. Quach, and M. A. Rizzacasa, ‘Total Synthesis of 8-Deshydroxyajudazol B’, Org. Lett., 2011, 13, 1964–1967.
56. J. E. Lynch, S. D. Zanatta, J. M. White, and M. A. Rizzacasa, ‘Stereoselective Total Synthesis of (-)-Spirofungin A by Utilising Hydrogen-Bond Controlled Spiroketalisation’, Chem. Eur. J., 2011, 17, 297–304.
2010
55. M. El Sous, D. Ganame, P. Tregloan, and M. Rizzacasa, ‘Total Synthesis of (-)-Reveromycin A via a Hetero-Diels-Alder Approach’, Synthesis (Stuttg)., 2010, 2010, 3954–3966.
54. C. Gunawan and M. A. Rizzacasa, ‘Mulberry Diels-Alder Adducts: Synthesis of Chalcomoracin and Mulberrofuran C Methyl Ethers’, Org. Lett., 2010, 12, 1388–1391.
2009
53. M. A. Rizzacasa and A. Pollex, ‘The hetero-Diels-Alder approach to spiroketals’, Org. Biomol. Chem., 2009, 7, 1053-1059.
52. T. E. Adams, M. El Sous, B. C. Hawkins, S. Hirner, G. A. Holloway, M. L. Khoo, D. J. Owen, G. P. Savage, P. J. Scammells, and M. A. Rizzacasa, ‘Total synthesis of the potent anticancer Aglaia metabolites (-)-silvestrol and (-)-episilvestrol and the active analogue (-)-4-desmethoxyepisilvestrol’, J. Am. Chem. Soc., 2009, 131, 1607–1616.
2008
51. J. T. Feutrill, M. J. Lilly, J. White, and M. A. Rizzacasa, ‘Asymmetric total synthesis of the myxobacteria metabolites crocacins A-D’, Tetrahedron, 2008, 64, 4880–4895.
2007
50. , , G. A. Holloway, D. J. Owen, P. J. Scammells, and M. A. Rizzacasa, ‘Total synthesis of (-)-episilvestrol and (-)-silvestrol.’, Angew. Chem. Int. Ed., 2007, 46, 7835–7838.
49. D. Ganame, T. Quach, C. Poole, and M. A. Rizzacasa, ‘Synthesis of the C9-C29 fragments of ajudazols A and B’, Tetrahedron Lett., 2007, 48, 5841–5843.
48. S. C. Zammit, V. Ferro, E. Hammond, and M. A. Rizzacasa, ‘Enantiospecific synthesis of the heparanase inhibitor (+)-trachyspic acid and stereoisomers from a common precursor’, Org. Biomol. Chem., 2007, 5, 2826–2834.
2006
47. J. Bunte, A. N. Cuzzupe, A. Daly, and M. A. Rizzacasa, ‘Formal Total Synthesis of (+)-Zaragozic Acid C through an Ireland-Claisen Rearrangement’, Angew. Chem. Int. Ed., 2006, 45, 6376–6380.
46. M. El Sous, D. Ganame, S. D. Zanatta, and M. A. Rizzacasa, ‘Total synthesis of spiroketal containing natural products: kinetic vs. thermodynamic approaches’, ARKIVOC, 2006, 7, 105–119.
2005
45. T. A. Munro, G. W. Goetchius, B. L. Roth, T. A. Vortherms, and M. A. Rizzacasa, ‘Autoxidation of salvinorin A under basic conditions’, J. Org. Chem., 2005, 70, 10057–10061.
44. M. Sous and M. A. Rizzacasa, ‘Biomimetic synthesis of the novel 1,4-dioxanyloxy fragment of silvestrol and episilvestrol’, Tetrahedron Lett., 2005, 46, 293–295.
43. S. C. Zammit, J. White, and M. A. Rizzacasa, ‘Enantiospecific synthesis of (+)-trachyspic acid’, Org. Biomol. Chem., 2005, 3, 2073–2074.
42. T. A. Munro, M. A. Rizzacasa, B. L. Roth, B. Toth, and F. Yan, ‘Studies toward the pharmacophore of salvinorin A, a potent kappa opioid receptor agonist’, J Med Chem, 2005, 48, 345–348.
2004
41. S. D. Zanatta, J. M. White, and M. A. Rizzacasa, ‘Total synthesis of the proposed structure for spirofungin B: a reassignment of the stereochemistry’, Org. Lett., 2004, 6, 1041–1044.
40. M. El Sous, D. Ganame, P. A. Tregloan, and M. A. Rizzacasa, ‘Total synthesis of (-)-reveromycin A’, Org. Lett., 2004, 6, 3001–3004.
39. F. Hilli, J. M. White, and M. A. Rizzacasa, ‘Formal total synthesis of (-)-apicularen A via transannular conjugate addition’, Org. Lett., 2004, 6, 1289–1292.
2003
38. A. K. Bigham, T. A. Munro, M. A. Rizzacasa, and R. M. Robins-Browne, ‘Divinatorins A-C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum’, J. Nat. Prod., 2003, 66, 1242–1244.
37. A. N. Cuzzupe, R. Di Florio, J. M. White, and M. A. Rizzacasa, ‘Enantiospecific synthesis of the phospholipase A2 inhibitors (-)-cinatrin C1 and (+)-cinatrin C3’, Org. Biomol. Chem., 2003, 1, 3572–3577.
36. T. A. Munro and M. A. Rizzacasa, ‘Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A’, J. Nat. Prod., 2003, 66, 703–705.
35. G. A. Holloway, H. M. Hügel, and M. A. Rizzacasa, ‘Formal total synthesis of salicylihalamides A and B’, J. Org. Chem., 2003, 68, 2200–2204.
34. I. R. Czuba, S. C. Zammit, and M. A. Rizzacasa, ‘Total synthesis of the marine sponge metabolites (+)-rottnestol, (+)-raspailol A and (+)-raspailol B’, J. Am. Chem. Soc., 2003, 1, 2044–2056.
33. D. Warren, G. Dyson, F. Grieser, J. Perera, G. Stevens, and M. A. Rizzacasa, ‘Characterisation of nickel (II) extraction by 2-hydroxy-5-nonylacetophenone oxime (LIX 84) in a micellar phase’, Colloids Surfaces A Physicochem. Eng. Asp., 2003, 227, 49–61.
32. J. T. Feutrill and M. A. Rizzacasa, ‘Total Synthesis of (+)-Crocacin A’, Aust. J. Chem., 2003, 56, 783–785.
2002
31. A. N. Cuzzupe, R. Di Florio, and M. A. Rizzacasa, ‘Enantiospecific synthesis of the phospholipase A2 inhibitor (-)-cinatrin B’, J. Org. Chem., 2002, 67, 4392–4398.
30. F. Hilli, J. White, and M. A. Rizzacasa, ‘Synthesis of the core of apicularen A by transannular conjugate addition’, Tetrahedron Lett., 2002, 43, 8507–8510.
29. J. T. Feutrill, M. J. Lilly, and M. A. Rizzacasa, ‘Total synthesis of (+)-crocacin D’, Org. Lett., 2002, 4, 525–527.
2001
28. A. N. Cuzzupe, C. A. Hutton, M. J. Lilly, R. K. Mann, K. J. McRae, S. C. Zammit, and M. A. Rizzacasa, ‘Total synthesis of the epidermal growth factor inhibitor (-)-reveromycin B’, J. Org. Chem., 2001, 66, 2382–2393.
2000
27. J. T. Feutrill, M. J. Lilly, and M. A. Rizzacasa, ‘Total synthesis of (+)-crocacin C’, Org. Lett., 2000, 2, 3365–3367.
26. A. N. Cuzzupe, C. A. Hutton, M. J. Lilly, R. K. Mann, M. A. Rizzacasa, and S. C. Zammit, ‘Total synthesis of (-)-reveromycin B’, Org. Lett., 2000, 2, 191–194.
25. M. El Sous and M. A. Rizzacasa, ‘Hetero-Diels-Alder synthesis of the spiroketal fragment of reveromycin A’, Tetrahedron Lett., 2000, 41, 8591–8594.
24. J. T. Feutrill, G. A. Holloway, F. Hilli, H. M. Hügel, and M. A. Rizzacasa, ‘Synthetic studies on the salicylihalamides: macrolactone formation via ring closing metathesis vs macrolactonization’, Tetrahedron Lett., 2000, 41, 8567–8572.
23. R. Di Florio and M. A. Rizzacasa, ‘Synthesis of the Syributins and Formal Total Synthesis of Syringolide 1.’, Aust. J. Chem., 2000, 53, 327–331.
1999
22. I. R. Czuba and M. A. Rizzacasa, ‘Total synthesis of (+)-rottnestol’, J. Chem. Soc. Chem. Common., 1999, 1999, 1419–1420.
1998
21. R. Di Florio and M. A. Rizzacasa, ‘Synthesis of 2,2-Disubstituted Furanoid Natural Products: Total Synthesis of Sphydrofuran’, J. Org. Chem., 1998, 63, 8595–8598.
20. R. K. Mann, J. Parsons, and M. A. Rizzacasa, ‘Towards the synthesis of the squalestatins/zaragozic acids: Synthesis of an advanced intermediate and introduction of the C-1 sidechain’, J. Chem. Soc. Perkin Trans. 1, 1998, 1998, 1283–1294.
1997
19. K. J. McRae and M. A. Rizzacasa, ‘Synthetic Studies toward the Reveromycins: Asymmetric Synthesis of the Spiroketal Segment of Reveromycin B’, J. Org. Chem., 1997, 62, 1196–1197.
1996
18. P. Chau, I. R. Czuba, M. A. Rizzacasa, G. Bringmann, K. P. Gulden, and M. Schäffer, ‘Convergent Synthesis of Naphthylisoquinoline Alkaloids: Total Synthesis of (+)-O-Methylancistrocline’, J. Org. Chem., 1996, 61, 7101–7105.
1995
17. B. Leighton and M. A. Rizzacasa, ‘Formal Synthesis of (-)-O-Methylancistrocladine’, J. Org. Chem., 1995, 60, 5702–5705.
16. R. Gable, R. Martin, and M. A. Rizzacasa, ‘A synthetic approach to (+)-dioncophylline C.’, Aust. J. Chem., 1995, 48, 2013–2022.
1994
15. J. Parsons and M. A. Rizzacasa, ‘Synthesis of the C-l Sidechain of the Squalestatins and Zaragozic Acid A.’, Tetrahedron Lett., 1994, 35, 8075-8075.
14. L. Mcvinish and M. A. Rizzacasa, ‘Synthetic studies towards the squalestatins and zaragozic acids’, Tetrahedron Lett., 1994, 35, 923–926.
1993
13. R. Ireland, R. Meissner, and M. A. Rizzacasa, ‘Convergent synthesis of polyether ionophore antibiotics: protective manipulation and synthesis of monensin A’, J. Am. Chem. Soc., 1993, 115, 7166–7172.
12. R. Ireland, J. Armstrong III, J. Lebreton, R. Meissner, and M. A. Rizzacasa, ‘Convergent synthesis of polyether ionophore antibiotics: synthesis of the spiroketal and tricyclic glycal segments of monensin’, J. Am. Chem. Soc., 1993, 115, 7152–7165.
1991
11. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the alkaloids of the ancistrocladaceae. Part 3. The total synthesis of (-)-ancistrocladinine: control of the diastereoisomer excess in the synthesis of axially chiral biaryls’, J. Chem. Soc. Perkin Trans. 1, 1991, 11, 2773–2781.
10. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the alkaloids of the ancistrocladaceae: control of the diastereoisomer excess in the synthesis of axially chiral biaryls: a synthesis of (-)-ancistrocladinine’, J. Chem. Soc. Chem. Commun., 1991, 5, 278–280.
9. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the naphthyl-isoquinoline alkaloids. Part 1. Dehydroancistrocladisine’, J. Chem. Soc. Perkin Trans. 1, 1991, 4, 841–844.
8. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the naphthyl-isoquinoline alkaloids. Part 2. The total synthesis of (-)-O-methylancistrocladine and (+)-O-methylhamatine and their enantiomers’, J. Chem. Soc. Perkin Trans. 1, 1991, 4, 845–854.
1990
7. M. A. Rizzacasa, M. V Sargent, B. W. Skelton, and A. H. White, ‘The Stereoisomers of 5-Bromo-6, 8-dimethoxy-1, 2, 3-trimethyl-1, 2, 3, 4-tetrahydroisoquinoline. X-Ray Crystal of the trans Isomer’, Aust. J. Chem., 1990, 43, 78–86.
6. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the alkaloids of the ancistrocladaceae:(-)-O-methylancistrocladine and (+)-O-methylhamatine’, J. Chem. Soc. Chem. Commun., 1990, 12, 894–896.
1989
5. M. A. Rizzacasa and M. V Sargent, ‘Synthetic approaches to the alkaloids of the ancistrocladacea: dehydroancistrocladisine’, J. Chem. Soc. Chem. Commun., 1989, 5, 301–302.
1988
4. M. A. Rizzacasa and M. V Sargent, ‘The synthesis of desertorin C, a bicoumarin from the fungus Emericella desertorum’, J. Chem. Soc. Perkin Trans. 1, 8, 2425–2428, 1988.
3. M. A. Rizzacasa and M. V Sargent, ‘The synthesis of stypandrol, a toxic binaphthalenetetrol isolated from stypandra imbricate: new syntheses of dianellidin and stypandrone’, Aust. J. Chem., 1988, 41, 1087–1097.
1987
2. M. A. Rizzacasa and M. V Sargent, ‘The structure and synthesis of nepenthone-A, a naphthoquinone from Nepenthes rafflesiana’, J. Chem. Soc. Perkin Trans. 1, 1987, 0, 2017–2022.
1. M. A. Rizzacasa and M. V Sargent, ‘The Wittig Reaction of 2-t-Butoxycarbonyl-1-methoxycarbonylethylidenetriphenylphosphorane: A Surrogate for the Stobbe Reaction’, Aust. J. Chem., 1987, 40, 1737–1743.